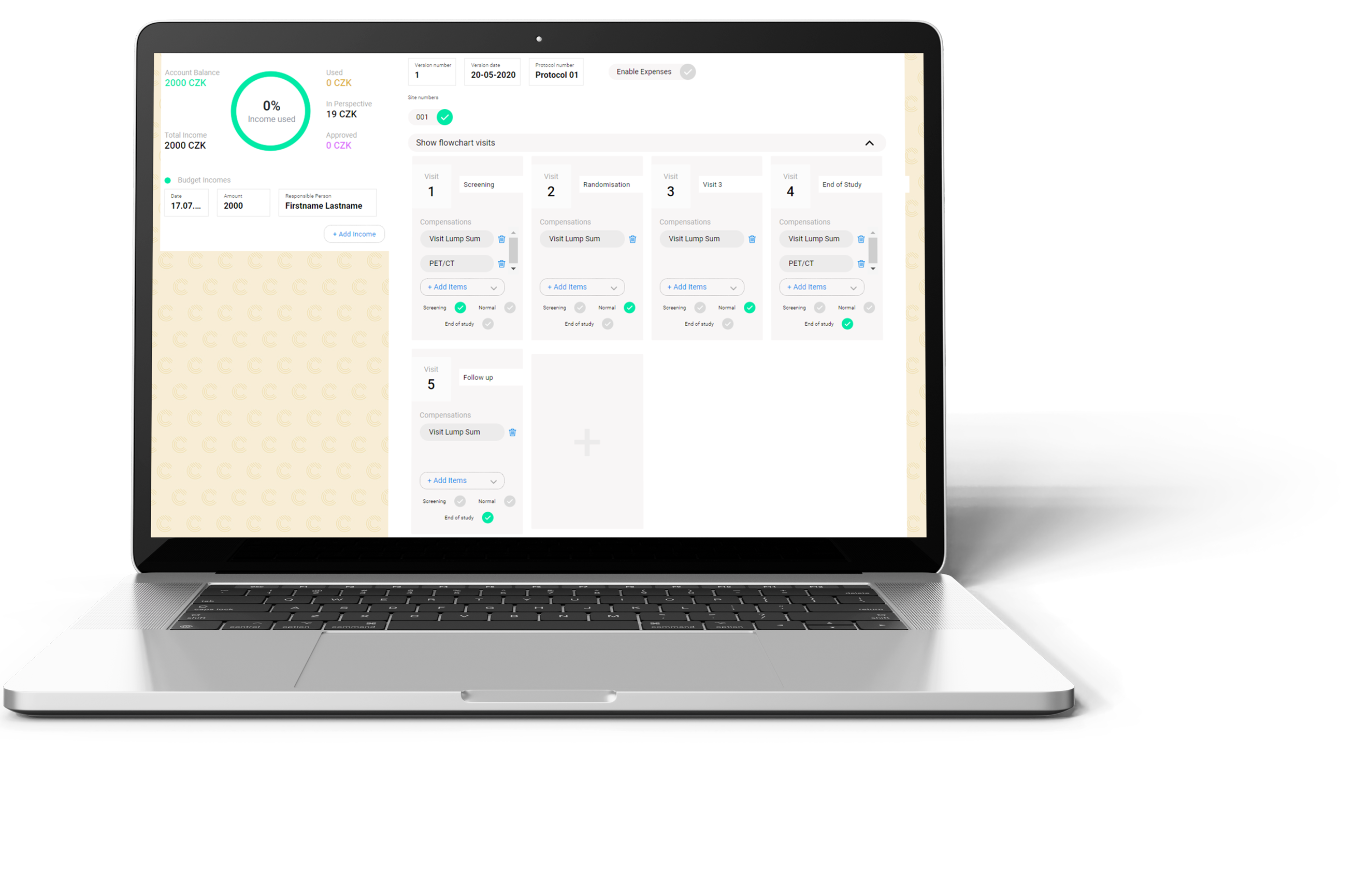
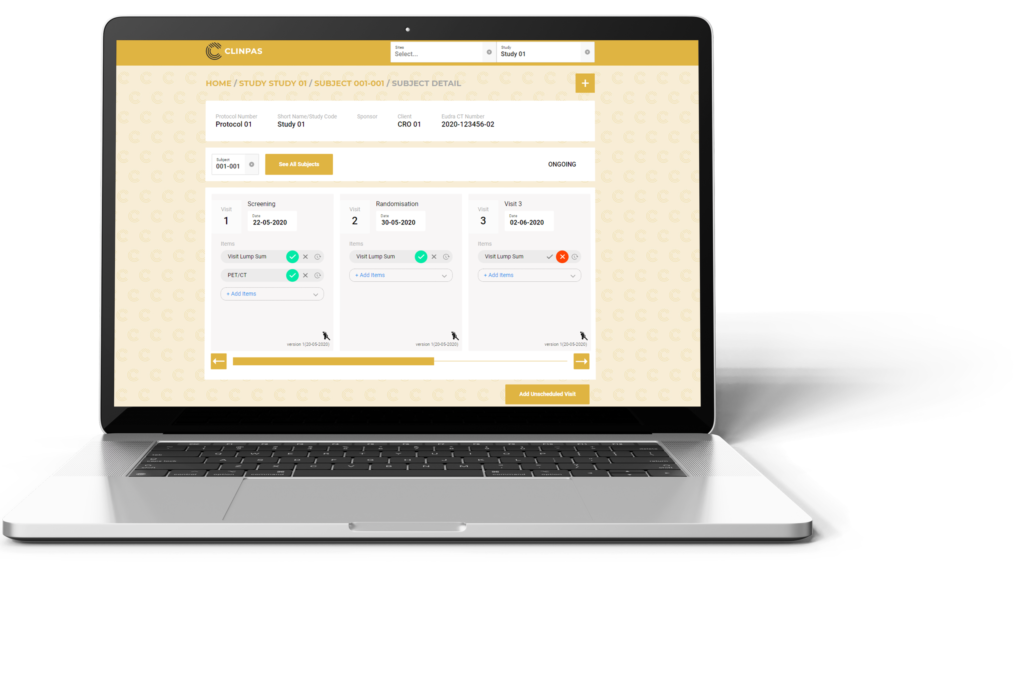
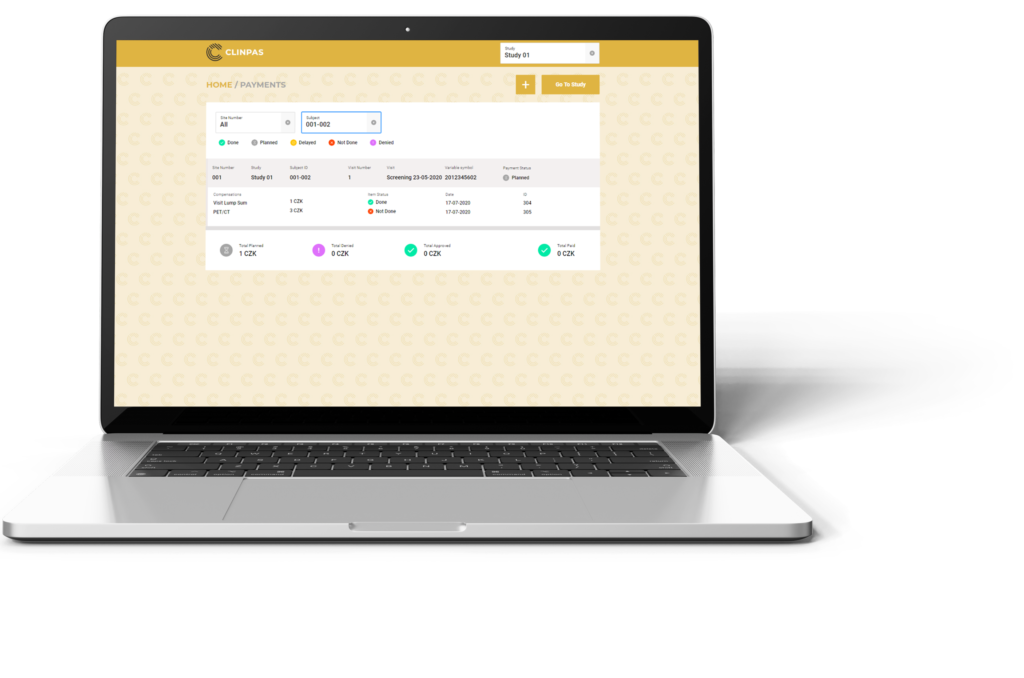
We have developed a unique online platform CLINPAS® for processing subject reimbursement in clinical trials. CLINPAS® enables a tailor-made configuration to each study protocol and reimbursement specifics, thus enabling an automated payment based on just a simple click by site staff confirming the milestone in the system.
CLINPAS® helps Sponsors, CROs and Investigators to make clinical trial subject reimbursement straight forward and easy.
Data confidentiality and security are kept as a matter of course.